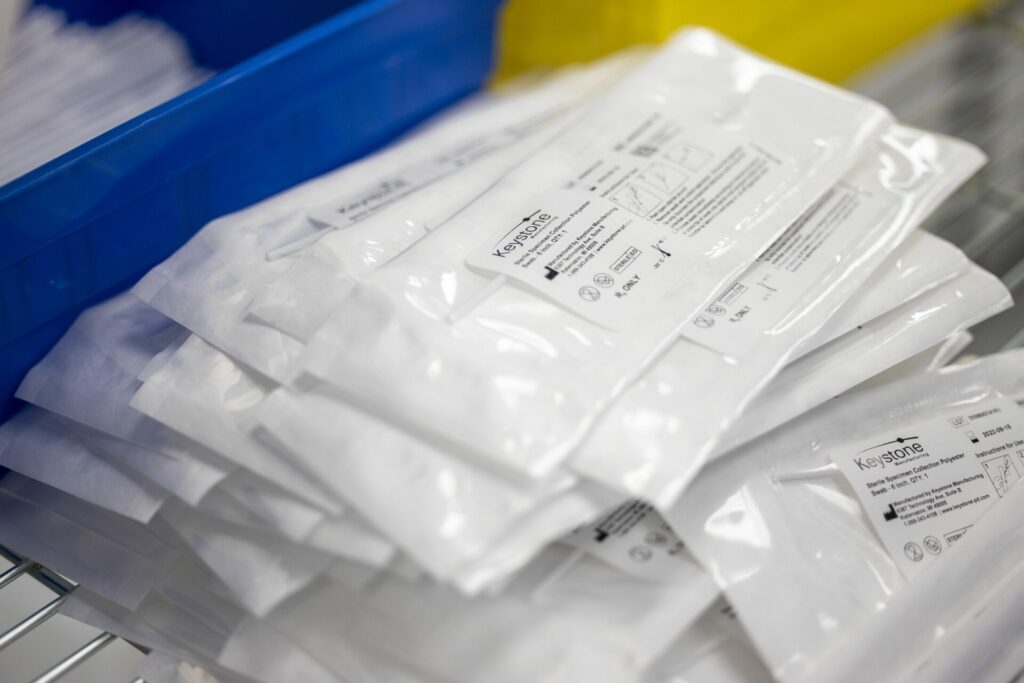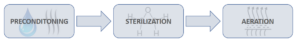
In the world of medical devices, the term “EtO” (or EO) is commonly used, especially in reference to single-use sterile devices. EtO stands for Ethylene Oxide and this is one of the most common methods used to sterilize medical devices. The packaging for devices intended to be EtO sterilized must include some type of permeable barrier such as Tyvek® to allow the gas to pass through the packaging and contact the device. The EtO sterilization process involves key steps such as pre-conditioning, sterilization, and aeration. During preconditioning, the product is subjected to a warm, humid environment to increase the moisture content in the packaging prior to EtO, which makes the EtO more effective, and warms the load. During the sterilization cycle, there are a number of key steps with the primary step being the introduction of EtO gas into the sterilization chamber. After EtO exposure one or more vacuum and nitrogen or air washes remove most of the EtO from the load. Sterilization cycle parameters are typically customized for a given product, and always validated to ensure sterility is attained. After the sterilization cycle, the product is placed into an aeration room or cell, where remaining EtO gas is allowed to escape the product and packaging. The product is then ready for use in the field.
Keystone has developed an Ethelene Oxide based sterilization cycle that is adaptable to a variety of medical devices. We have cleared the path for product adoption by using a robust protocol and challenge devices. Once a product is adopted into the validation, Keystone can manage bioburden monitoring, scheduling and logistics for routine cycles, and review of records related to routine production. We can also coordinate product and package testing with our partners at third party laboratories across the United States.