Customer Product Transfer
Transferring a medical device program is not as simple as moving the physical assets to a new location and restarting production. There are many other items that need to be addressed to comply with regulations, onboard suppliers, and ensure ongoing robust quality and documentation. Equipment qualifications and packaging validations are often required as well. The scope of these items is driven by the device complexity, device classification, and other factors.
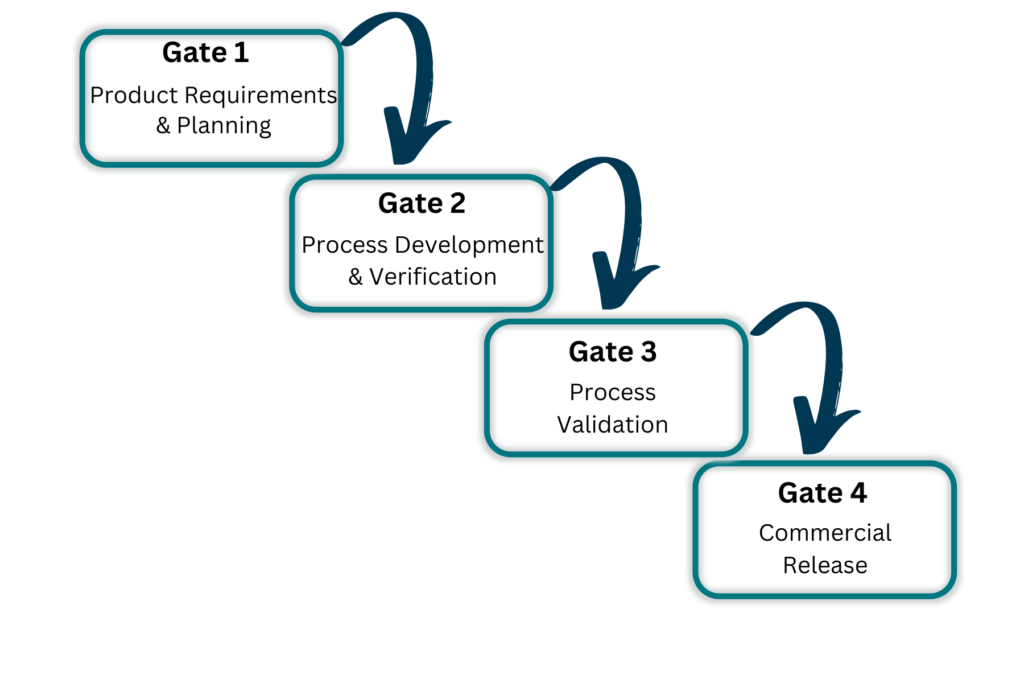
Keystone has developed the Customer Product Transfer process (CPT) to optimize manufacturing transfer programs. The CPT is a gate driven process that was developed based on many years of tribal knowledge in executing a wide range of manufacturing transfer programs. The CPT process helps ensure timelines are minimized and regulatory requirements are met.



Fast Track EtO Sterilization Validations
The words “Fast Track” and “EtO” are not often heard in our industry, especially in the last few years. With continually mounting pressures on the Ethylene Oxide Sterilization (EtO) process for lower emissions, process improvements, and certain facilities being shut down, getting EtO cycles created, validated, and in production has not been a Fast Track journey. In addition to lead times being significantly extended for the validation process, the overall costs have skyrocketed as well.
Keystone offers a solution that significantly reduces the validation timeline and cost. The team at Keystone has developed a proprietary, validated EtO cycle based on a number of challenge devices. The challenge devices were chosen based on their complexity and torturous path with the goal of creating a robust cycle that can be applied to a wide range of products. As Keystone clients look to get their products EtO sterilized, in most cases, the Keystone cycle can be employed by utilizing what is known as a product adoption (vs a full validation). The product adoption process is far more efficient in terms of cost and timeline as compared to a full validation. The number of validation cycles, testing, and documentation are all minimized when a product adoption is possible.
The Keystone cycle is also flexible. Whether your volumes demand one pallet per load, or several, we can tailor the process to your needs. This also helps reduce cost and improve efficiency.
Medical Device
Manufacturing
Specialty Areas
- Wound Care
- Diagnostics
- Imaging
- Therapeutic Devices
- Electro-mechanical
- Orthopedic Systems
Contract Manufacturing
- ISO 13485:2016 and FDA Registered
- Cleanroom Assembly
- Kitting
- Packaging
- Sterilization Management
- Converting
- Warehousing & Distribution
How We Do It
Our proven
Process
How We Provide Solutions
Our customers trust us to provide creative solutions to complex problems. We do this by offering a comprehensive set of services with our top-notch experienced team and by utilizing our extensive in-house resources and via our wide-ranging sourcing capabilities. It’s real world innovation and program management to meet project deliverables.